COVID-19 Testing Workflow Products
Zymo Research has supported global COVID-19 testing efforts since the beginning of the pandemic with sample collection reagents, viral extraction kits, and COVID-19 tests. In addition to a FDA EUA RT-PCR workflow, and the first 510(k)-cleared transport medium for COVID-19 testing, we also offer a wide range of custom solutions.
510(k)-Cleared Transport Medium for COVID-19 Testing

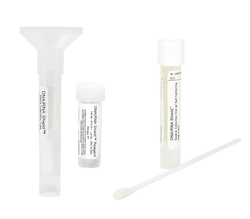
DNA/RNA Shield Collection Tube w/ Swab
The DNA/RNA Shield collection tube is intended for the stabilization and inactivation of upper and lower respiratory human specimens suspected of containing SARS-CoV-2.
DNA/RNA Shield SafeCollect Devices
Sample collection devices for simple, non-invasive unsupervised testing. SafeCollect’s patented tube features a safety seal that prevents users from spilling, ingesting, or being exposed to the stabilization solution. A unique safety seal is punctured after adding a sample to the tube, releasing the stabilization solution.
Learn More
High-Throughput SARS-CoV-2 RNA Extraction
Quick-DNA/RNA Viral Magbead Kits
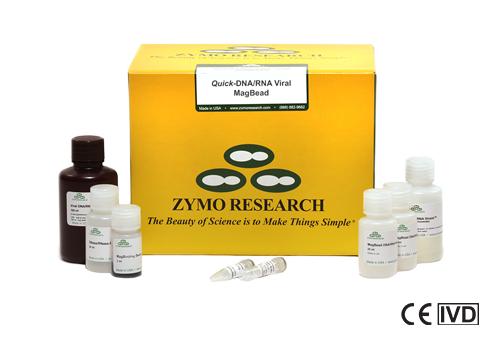
The Quick-DNA/RNA Viral Magbead Kits (R2140-E, R2141-E) are Emergency Use Authorized and CE-IVD for diagnostic use. The kits are used in Zymo Research’s SARS-CoV-2 workflow and are an ideal high-throughput solution for early viral detection.
Learn MoreSensitive, High-Throughput COVID-19 Detection
Quick SARS-CoV-2 Multiplex Kit
The Quick SARS-CoV-2 Multiplex Kit is a highly sensitive and specific molecular test with improved throughput capability. The ready-to-use reagents and the competitive pricing make the Quick SARS-CoV-2 Multiplex Kit the test of choice for any laboratory. The Quick SARS-CoV-2 Multiplex Kit is CE-IVD marked.
Learn MoreZymo Research's FDA EUA SARS-CoV-2 Workflow
The end-to-end workflow under the EUA authorization consists of sample collection, sample preparation, and real-time RT-PCR analysis. Intended use of the products mentioned in the graphic above may vary.
Organizations in need of reagents or complete workflows for consolidated COVID-19 testing should inquire here:
covid19requests@zymoresearch.com
Zymo Research Technology Proudly Supports COVID-19 Testing Workflows
Molecular Transport Media (MTM) Technology That Supports Monkeypox and COVID Workflows
FAQ
The FDA has recently granted Zymo 510(k) clearance for the DNA/RNA Shield collection tube as a class II medical device, intended for the stabilization and inactivation of upper and lower respiratory human specimens suspected of containing SARS-CoV-2.
Additionally, several of Zymo products have been authorized by the FDA for emergency use (Emergency Use Authorization) during the COVID-19 pandemic. These include the DNA/RNA Shield collection tube w/ swab, DNA/RNA Shield saliva/sputum collection kit, Quick-DNA/RNA Viral MagBead Extraction Kit for sample extraction and the Quick SARS-CoV-2 rRT-PCR Test Kit for the sensitive detection of SARS-CoV-2 virus in respiratory samples.
Zymo products (sample collection devices and viral extraction kits) are also available as a CE marked in Vitro diagnostic device (CE-IVD) and have been commercialized in the European Union and other applicable countries.
Feel free to contact us, for additional information or assistance: covid19requests@zymoresearch.com.
We offer a variety of viral extraction kit formats for both DNA and RNA. For high-throughput, automatable options, we highly recommend our Quick-DNA/RNA Viral MagBead Kit. Our technical support team is standing by to assist with any product inquiries: tech@zymoresearch.com.
For high-throughput, automatable options, we recommend our CE-IVD marked Quick-DNA/RNA Viral MagBead Kit. Scripts are available for Tecan, Hamilton, and KingFisher automated platforms. For other platforms, including Illumina's® COVIDSeq©, resources and support are available. Our dedicated automation team is standing by and ready to help. Please contact our automation specialists for further support and further automation information: automation@zymoresearch.com.
Yes. We have a dedicated team of specialists readily available to assist you with questions regarding product availability, product recommendations, and regulatory requirements. Please email covid19requests@zymoresearch.com.