Quick SARS-CoV-2 Multiplex Kit
(For COVID-19 Detection)
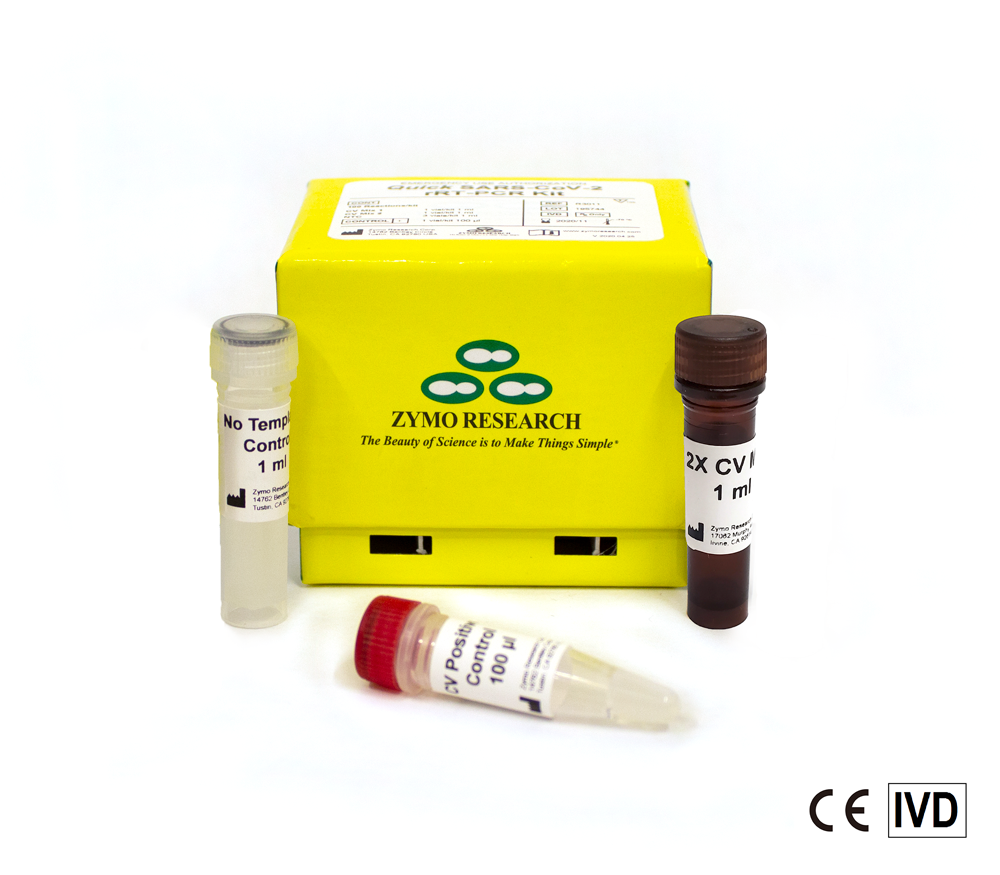
Reliable, Affordable Testing Solutions for COVID-19
The Quick SARS-CoV-2 Multiplex Kit is a highly sensitive and specific molecular test with improved throughput capability. The ready-to use reagents and the competitive pricing make the Quick SARS -CoV-2 Multiplex Kit the test of choice for any laboratory. The Quick SARS-CoV-2 Multiplex Kit is CE-IVD marked. Zymo Research plans on submitting an FDA EUA application for the Quick SARS-CoV-2 Multiplex Kit and workflow for SARS-CoV-2 virus and COVID-19 detection.
Sensitivity
Extremely sensitive, with 100% viral detection at 10 GEC/reaction (167 GEC/ml of sample).
Specificity
Specific detection of SARS-CoV-2 and emerging strains. No cross-reactivity with other pathogens and viruses.
Controls
Robust control system that minimizes false negative results.
Simplicity
Ready-to-use reagents for simple set-up and minimal hands-on time. Results are generated in less than 2 hours.
Competitive Pricing
Affordable solution for every laboratory to allow better COVID-19 testing.
Flexibility
Compatible with Real-Time PCR instrumentation capable of detecting HEX/VIC and Quasar670/Cy5 fluorophores.*
Intended Use
Quick SARS-CoV-2 Multiplex Kit is a real-time RT-PCR test intended for the qualitative detection of nucleic acids from SARS-CoV-2 in upper respiratory specimens (such as nasal, nasopharyngeal, mid-turbinate or oropharyngeal swabs), and lower respiratory specimens (such as sputum, tracheal aspirates, and bronchoalveolar lavage) from patients suspected of COVID-19 by medical personnel. Testing is limited to diagnostic laboratories certified by health authorities to perform high complexity tests.
The test is intended for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable in upper and lower respiratory specimens during the acute phase of infection.
Positive results are indicative of the presence of SARS-CoV-2 RNA. Clinical observations, patient history and other diagnostic information are necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
The Quick SARS-CoV-2 Multiplex Kit should be used by laboratory personnel trained in the techniques of real-time RT-PCR assays.
Discuss Your Needs with Our Scientists
Learn More About Zymo Research’s COVID-19 Solutions
SARS-CoV-2 detection system
- Simple reaction set-up.
- 100% detection at 15 GEC/rxn.
- Results in < 1.5 hours after extraction.
- Includes CV Mix 1 (for SARS-CoV-2 detection), CV Mix 2 (internal human control), CV Positive Control and No Template Control.
*Adjustments of RT-PCR parameters may be necessary.
This test has not been FDA cleared or approved.