Quick SARS-CoV-2 rRT-PCR Kit
Authorized Under FDA EUA for COVID-19 Testing

Reliable, Affordable Testing Solutions for COVID-19
The Quick SARS-CoV-2 rRT-PCR Kit is for use under the FDA's Emergency Use Authorization (EUA) in conjunction with Zymo Research's sample collection and viral RNA purification systems. The test is ranked third in sensitivity on the FDA SARS-CoV-2 Reference Panel and is being used as reference material to assist other developers of SARS-CoV-2 nucleic acid-based amplification tests (NAATs). The kit is also CE-IVD certified for countries requiring this mark. In addition, Zymo Research has a secure, reliable supply of the Quick SARS-CoV-2 rRT-PCR Kit and COVID-19 testing reagents.
Sensitivity
Extremely sensitive, with 100% viral detection at 15 GEC/reaction (250 GEC/ml of sample).
Specificity
Simultaneous detection of three conserved viral regions specific for SARS-CoV-2 and Sarbecoviruses.
Controls
Robust control system that minimizes false negative results.
Simplicity
Ready-to-use reagents for simple set-up and minimal hands-on time and results generated in less than 1.5 hours.
Competitive Pricing
Affordable solution with flexible pricing.
Flexibility
Compatible with Real-Time PCR instrumentation capable of detecting HEX/VIC and Quasar670/Cy5 fluorophores.
Intended Use
Quick SARS-CoV-2 rRT-PCR Kit is a real-time RT-PCR test intended for the qualitative detection of nucleic acids from SARS-CoV-2 in upper respiratory specimens (such as nasal, nasopharyngeal, mid-turbinate or oropharyngeal swabs), and lower respiratory specimens (such as sputum, tracheal aspirates, and bronchoalveolar lavage) from patients suspected of COVID-19 by their healthcare provider. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high complexity tests.
Results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable in upper and lower respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2 RNA. Clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
The Quick SARS-CoV-2 rRT-PCR Kit is intended for use by qualified clinical laboratory personnel specifically instructed and trained in the techniques of real-time RT-PCR assays. The Quick SARS-CoV-2 rRT-PCR Kit is only for use under the Food and Drug Administration’s Emergency Use Authorization.
Quick SARS-CoV-2 rRT-PCR Kit is a real-time RT-PCR test intended for the qualitative detection of nucleic acids from SARS-CoV-2 in upper respiratory specimens (such as nasal, nasopharyngeal, mid-turbinate or oropharyngeal swabs), and lower respiratory specimens (such as sputum, tracheal aspirates, and bronchoalveolar lavage) from patients suspected of COVID-19 by medical personnel. Testing is limited to diagnostic laboratories certified by health authorities to perform high complexity tests.
The test is intended for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable in upper and lower respiratory specimens during the acute phase of infection.
Positive results are indicative of the presence of SARS-CoV-2 RNA. Clinical observations, patient history and other diagnostic information are necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
The Quick SARS-CoV-2 rRT-PCR Kit should be used by laboratory personnel trained in the techniques of real-time RT-PCR assays.
Learn more about Zymo Research’s COVID-19 FDA EUA Workflow:
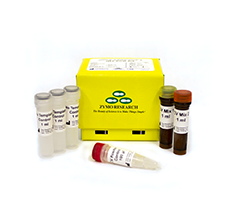
SARS-CoV-2 detection system
- Simple reaction set-up.
- 100% detection at 15 GEC/rxn.
- Results in < 1.5 hours after extraction.
- Includes CV Mix 1 (for SARS-CoV-2 detection), CV Mix 2 (internal human control), CV Positive Control and No Template Control.
Customer Feedback
This test has not been FDA cleared or approved. This test has been authorized by the FDA under an EUA for use by authorized laboratories. This test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens. This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner. Detection of SARS-CoV-2 RNA may be affected by sample collection methods, patient factors, and/or stage of infection.